Abstract
Introduction
Venetoclax, a BCL-2 specific inhibitor, used in combination with azacitidine in patients with newly-diagnosed acute myeloid leukemia (AML) who were ineligible for intensive chemotherapy treatment, has shown high response rate and overall survival compared with azacitidine monotherapy (Di Nardo et al. N Engl J Med 2020; 383:617-629). Moreover, venetoclax in combination with hypomethylating agents or with low-dose cytarabine is being explored in other settings being frequently used in relapsed/refractory (R/R) AML.
Aims: We performed a retrospective study of patients diagnosed with R/R AML receiving azacitidine combinations in the Catalan Institute of Oncology (ICO) in order to determine the efficacy and safety of the combination.
Methods
We analyze 35 patients diagnosed with R/R AML at 4 hospitals belonging to ICO in Spain, treated with venetoclax (400mg/24h; initial daily dose of 100mg with a 3-day ramp-up to target dose of 400mg) in combination with hypomethylating agents (azacitidine 75mg/m 2 or decitabine 20mg/m 2) or low-dose cytarabine (20mg/m 2) from May 2019 to Agost 2021. Event was defined as death, refractoriness to treatment or progressive disease.
Results
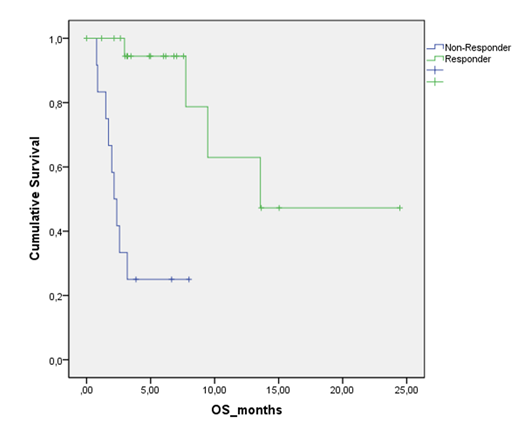
Median age was 72 years (range 44-82). Seventeen (43%) patients had high-risk AML according to ELN 2017. Five (14%) patients received venetoclax in combination with decitabine, 20 (57%) patients with azacitidine, and 10 (29%) patients with low-dose cytarabine. The median number of cycles received was 3 (range 1-25). Early mortality in the first 30 days was 5.7% (2 patients). Overall response rate (ORR) was 58%. Complete remission (CR) rate was 48% and 10% partial remission. Seventeen patients (43%) needed venetoclax dosage adjustments due to hematologic toxicity. Median time to response was 2 months. Four patients (10%) were transitioned to allogeneic stem cell transplantation. The median OS was 9.5 months (95% C.I. 2.6-16.2). Response to treatment after 3-4 cycles, discriminate two groups patients with an OS of 13.57 months in those patients who achieved CR or PR vs 2.1 months in non-responders (p<0001). Thirteen patients died as a result of infection (8.2%), disease progression (4.1%), and bleeding (1.3%)
Summary/Conclusions
Our study showed that real-world experience of treating patients with R/R AML with venetoclax in combination with hypomethylating agents or low-dose cytarabine is feasible, well tolerated with a rapid and promising response rate and low toxicity profile. Moreover, rapid responses shown with the combination, allow us to identify those patients who may benefit from this approach.
Sureda: Mundipharma: Consultancy; Bluebird: Membership on an entity's Board of Directors or advisory committees; Roche: Other: Support for attending meetings and/or travel; GSK: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal